Overview
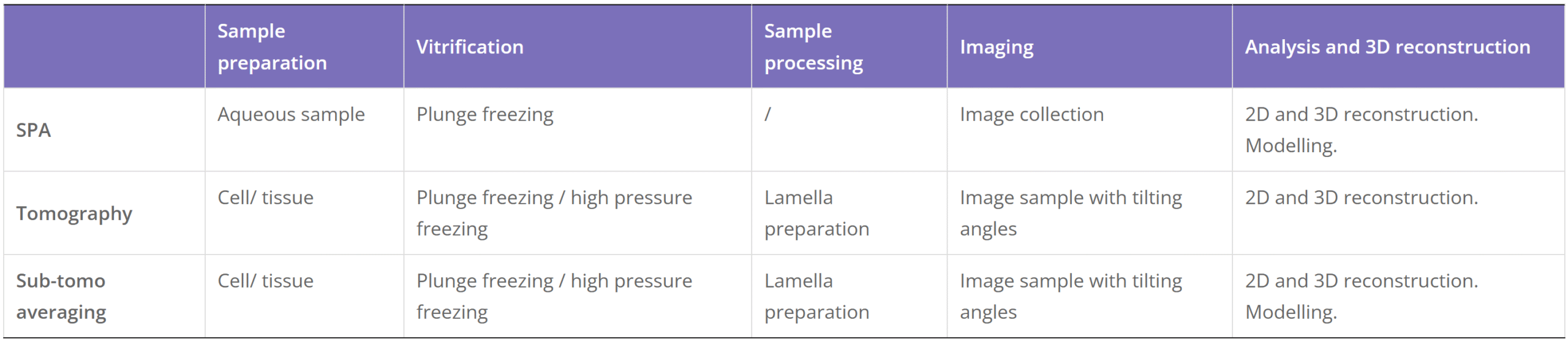
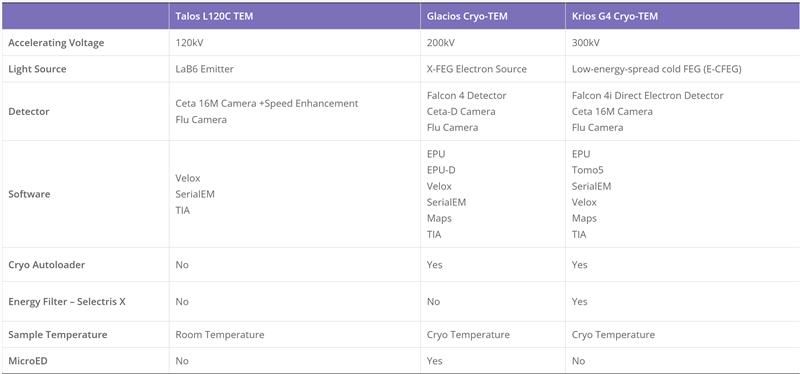
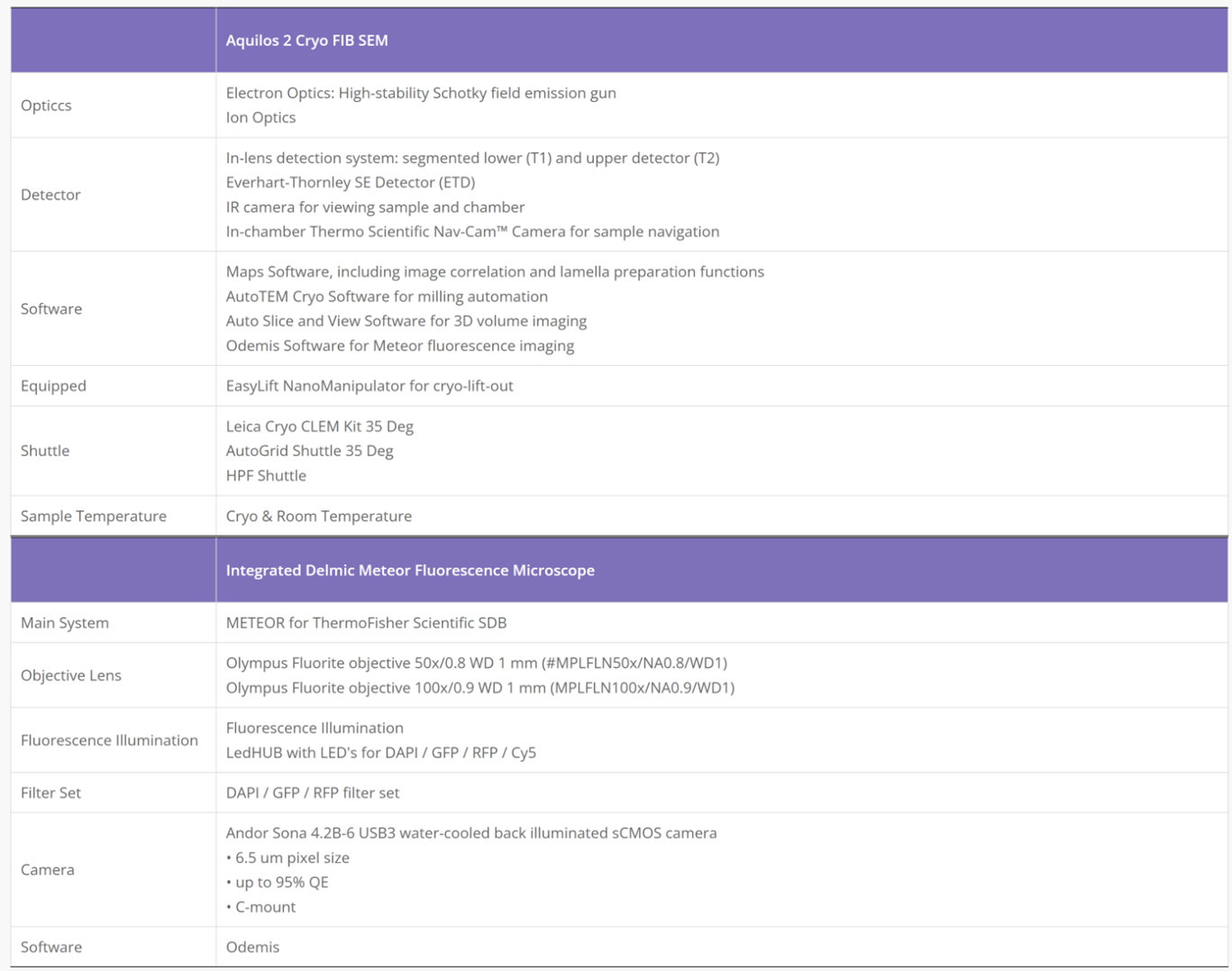
Cryo-Electron Microscopy is the imaging of specimens frozen in vitreous ice and maintained at liquid nitrogen temperature using Electron Microscopes. In this method, specimens can be studied in their native state without dyes or fixatives, enabling the analysis of fine cellular structures, viruses, and proteins at molecular resolution. Despite being a decades-developed technique, Cryo-EM has been attracting interest since 2013 as a result of technological and algorithmic improvements that have driven a dramatic improvement in the resolution achievable using this technique (dubbed the ‘resolution revolution’). In 2017, the technique won the Nobel Prize in Chemistry.
The Cryo-EM technique is becoming the first choice of many structural biologists when analyzing the protein structure experimentally. As a technique for determining the atomic structure of macromolecules that neither crystallize nor are difficult to crystallize under certain conditions, Cryo-EM has the same level of resolution as X-ray crystallography. Cryo-EM is the best way to study cell architecture, large proteins, membrane-bound receptors, or complexes of macromolecules.